2007年VOA标准英语-US Health Agency Issues Warning on Children's C(在线收听)
Washington
21 August 2007
The U.S. Food and Drug Administration recently issued a warning that parents not give young children commercially sold cough and cold medicine, unless a doctor approves it first. The concern came after a two-year study of at least 1,500 children who were hospitalized after taking the syrup. VOA's Melinda Smith has more.
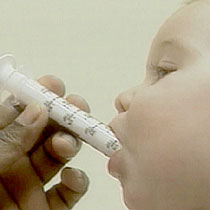 |
| An infant being given doctor-supervised medicine |
The warning from the U.S. Food and Drug Administration comes after many pediatricians were concerned that parents were not reading the fine print.
Dr. Lisa Thornton, from Schwab Rehabilitation Hospital in Chicago, Illinois, says parents should be cautious about what they give their children. "These are not harmless medications and they really do need to be dosed very appropriately and, as I said, minimally for very young children."
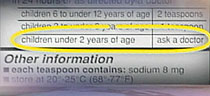 |
| Warning on a medicine label |
Dr. Janet Serwint of Johns Hopkins Children's Center in Baltimore, Maryland describes some of the side effects. "They can cause your heart to beat too fast. They can cause your blood pressure to be elevated."
An organization representing manufacturers of cough and cold medications insists the products are safe. It also agreed that a doctor should be consulted first.